Notwithstanding
considerable variations in daily food intake, animals uphold a remarkably
stable body weight, since overall caloric ingestion and expenditure are
exquisitely matched over long periods of time, by the process of energy
homeostasis.
David
E Cummings and Joost Overduin, 2007(152)
The
elegant ‘interconnected mechanisms’ by which the gastrointestinal (GI) tract
regulates food intake are a marvel of biology, but the redundancy (e.g.,
several hormones seem to have effects in food intake) of both GI (by means of hormones) and central
nervous system (CNS, by means of satiety/satiation signals) pathways governing
energy homeostasis poses formidable
challenges for scientists trying to take a clear glimpse of this machinery,
e.g. for designing anti-obesity and alike
pharmaceuticals.
In
essence, notwithstanding the astonishing advancements made over the past few
decades in unscrambling many of the molecular pathways involved in energy
(homeostasis) regulation, a rather cloudy understanding of “how all the pieces
fit together to function as an integrated system” is what can be found for the
most part in the scientific community; we discuss that in part II of the work,
in a single chapter divided in several sections for numerous imperative
hormones, e.g. cholecystokinin.
The
current work is divided into three parts: part I is regarding fundamentals of
physiology and mathematical modeling employed all over the work; part II is
more generic and concerns several hormones (what we have called a “web of
hormones”) and part III (divided into three chapters) is more specific,
concerning a single hormone (i.e., ghrelin). The core of the work is part III, and
to a certain extent part II, bearing mind we provide a literature review based
on papers scattered/dispersed all over the medical science literature.
The main
objective of this work is proposing a
mathematical model for ghrelin dynamics (Figure
68), a
model centered on the gastrointestinal
tract (stomach + small intestine, a two-compartment model), with daily-like
dynamics, short-term dynamics; and, simultaneously, proposing a prototype for a systems biology like model (Figure
38), a
model based on numerous hormones, for understanding mathematically food
intake/bodyweight control
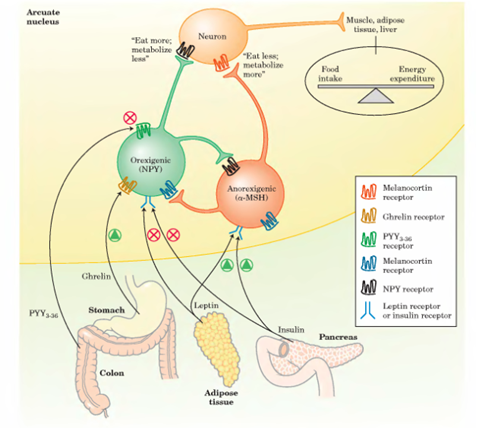
Ghrelin
is a quite powerful orexigenic hormone discovered in the late 1990s that
controls appetite and energy homeostasis, alongside leptin and other hormones still to be
investigated in depth by the medical sciences literature. Accordingly, we
provide a (simple) mathematical model, consisting of a set of ordinary differential
equations detailing ghrelin
dynamics combined to gastrointestinal signals due to meals. Numerical
simulations are able to replicate in silico available data from the literature;
additionally, we were able to fit a reduced version of the basal model to
experimental data. The model is developed as a module for a bigger potential multi-compartmental
structure, detailing food and energy homeostasis within a sort of "a web
of hormones" (see part II and the last chapter of part III). The present
contribute is to recommend a primary mathematical model for ghrelin dynamics
centered in the gastrointestinal tract,
with potentiality to be applied also for postabsorptive states, left mainly as
future works. We go on with the model by presenting mainly two variations,
further unfolding is left as future endevour: tastants and stochastic version.
We
test several optimization routines for the parameter estimation procedure,
hybrid algorithms (global + local search), for parameter estimation, based on
data published for humans (three meals a day). For all the routines, the best
is a hybrid composed of simulating annealing as global search and pattern
search as local search. In the objective function (sum of the squared errors,
SSE), we apply artificial neural networks (a two-layer feedforward neural
network) for generating new data from the data already published, a strategy adopted
to increase the data set. In the last part of the chapter about ghrelin
modeling (part III), we propose several prototypes for future works based on
the basal models; the model used for parameter estimation is a
“minimal/reduced” model; we also provide discussions and future works for the
minimal model and parameter estimation.
Key-words. Ghrelin;
leptin; mathematical modelling; food intake; appetite; parameter estimation.



No comments:
Post a Comment