This is a blog about an ongoing PhD research. The aim is designing a mathematical model for ghrelin, insulin and leptin, having glucose control as commonplace.
Welcome words
1. Introduction
Ghrelin, produced mainly in the stomach, is an appetite stimulating hormone, an powerful orexigenic bio-molecule; i.e. it triggers the need for food, our appetite. It has been discovered in 1999 by Japanese scientists, but largely spread-out by British groups, and so then it has been a quite important piece for taking in the workings of feeding patterns and behaviors.
Ghrelin is an amino acid peptide, related to growth hormone, which is secreted primarily in the stomach but is found throughout the gastrointestinal system and even in the hypothalamus and amygdala, among other sites, such as the heart and pancreas. Some claims that the name comes from Growth Hormone releasing, by shorting and gathering, we encounter ghrelin. But exactly how ghrelin exerts its effect is not clear, neither how it is produced, e.g. the complete profile for triggering ghrelin activation and inhibition.
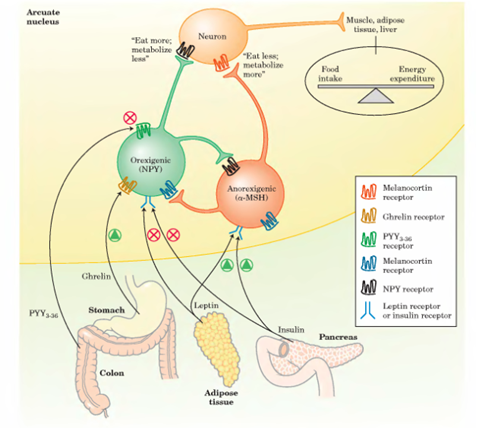
2. The complex process of eating
We can say that eating is one of the simplest activities, it is not necessary being an Einstein for eating with style. Nonetheless, within the body, it is a quite complex process, maybe amongst the most complex ones, given that we are still try to understand it properly for treating medical conditions such as obesity.
Defense
Ghrelin receptors in the brain
Ghrelin mathematical model (current)

This is the current model for ghrelin
Future works
Future works
Likely not for now
- Image processing;